Mercury (Hg) AMERICAN ELEMENTS
9.4: The Bohr Model - Atoms with Orbits is shared under a CK-12 license and was authored, remixed, and/or curated by Marisa Alviar-Agnew & Henry Agnew. LICENSED UNDER. Bohr's model suggests that each atom has a set of unchangeable energy levels, and electrons in the electron cloud of that atom must be in one of those energy levels.

Mercury Atom Project Atom project, Projects, Crafts for kids
In this way, it can be explained why mercury, for example, emits a specific energy spectrum to which specific wavelengths (colors) in the light spectrum belong. The figure below shows the emitted spectrum of a mercury-vapor lamp.. Although the Bohr model of the atom is a further development of Rutherford's atomic model, it also contains.

Mercury Atom Vector Graphic Download Free Vector Art, Stock Graphics
The Bohr Model. In 1913, a Danish physicist, Niels Bohr (1885-1962; Nobel Prize in Physics, 1922), proposed a theoretical model for the hydrogen atom that explained its emission spectrum.. neon; and (c) mercury. The strongest lines in the hydrogen spectrum are in the far UV Lyman series starting at 124 nm and below. The strongest lines in.

Complete Electron Configuration for Mercury (Hg)
Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. Bohr's model calculated the following energies for an electron in the shell, n : E ( n) = − 1 n 2 ⋅ 13.6 eV
Mercury stock illustration. Illustration of rendering 175797080
The Bohr model is a relatively primitive model of the hydrogen atom, compared to the valence shell model. As a theory, it can be derived as a first-order approximation of the hydrogen atom using the broader and much more accurate quantum mechanics and thus may be considered to be an obsolete scientific theory.

Mercury, atomic structure Stock Image C018/3761 Science Photo Library
Figure \(\PageIndex{2}\): The Bohr Model of the Hydrogen Atom (a). Similarly, the blue and yellow colors of certain street lights are caused, respectively, by mercury and sodium discharges. In all these cases, an electrical discharge excites neutral atoms to a higher energy state, and light is emitted when the atoms decay to the ground state.
Mercury (Hg) AMERICAN ELEMENTS
Bohr Model of all Elements (Diagrams + Chart) March 23, 2023 by Jay Bohr model of all Elements is mentioned in the chart below. Free Gift for you: Interactive Periodic Table Let me tell you how this Interactive Periodic Table will help you in your studies. 1).
PPT Mercury (Hg) PowerPoint Presentation, free download ID6592903
Slower electrons merely bounce off mercury atoms without losing any significant speed or kinetic energy. These experimental results proved to be consistent with the Bohr model for atoms that had been proposed the previous year by Niels Bohr. The Bohr model was a precursor of quantum mechanics and of the electron shell model of atoms. Its key.

Mercury Bohr Model Atom Necklace Sorcery Science
Bohr model, description of the structure of atoms, especially that of hydrogen, proposed (1913) by the Danish physicist Niels Bohr.The Bohr model of the atom, a radical departure from earlier, classical descriptions, was the first that incorporated quantum theory and was the predecessor of wholly quantum-mechanical models. The Bohr model and all of its successors describe the properties of.
Hg Mercury Element Information Facts, Properties, Trends, Uses and
The Bohr model is a neat but quite imperfect depiction of the inner workings of an atom before things got too muddled up by quantum principles.. It may orbit at the distance of Mercury, then.

Bohr Model Representation Mercury Atom Number Stock Vector (Royalty
Figure \(\PageIndex{2}\): The Bohr Model of the Hydrogen Atom (a). Similarly, the blue and yellow colors of certain street lights are caused, respectively, by mercury and sodium discharges. In all these cases, an electrical discharge excites neutral atoms to a higher energy state, and light is emitted when the atoms decay to the ground state.
Bohr Model Atomic Number Thorium Periodic Table Symbol Mercury
The Bohr model represents the particle nature of electrons. So, it's easy to see that the atom above contains two electrons. As we'll discuss later in the article, atomic electrons exist at specific energy levels. The Bohr model represents these energy levels as rings.

Related image Electron configuration, Mercury, Element
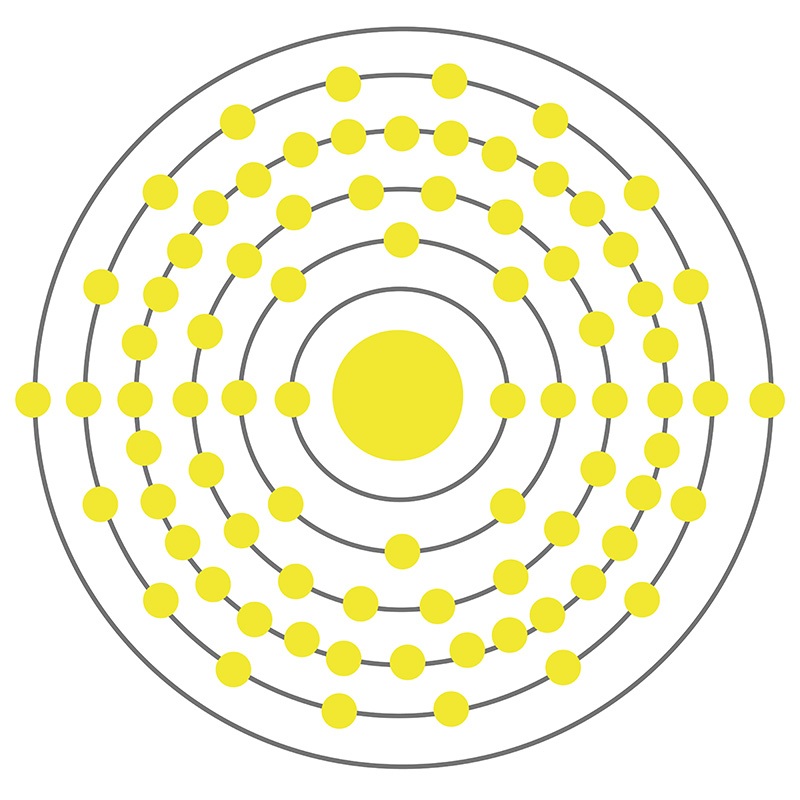
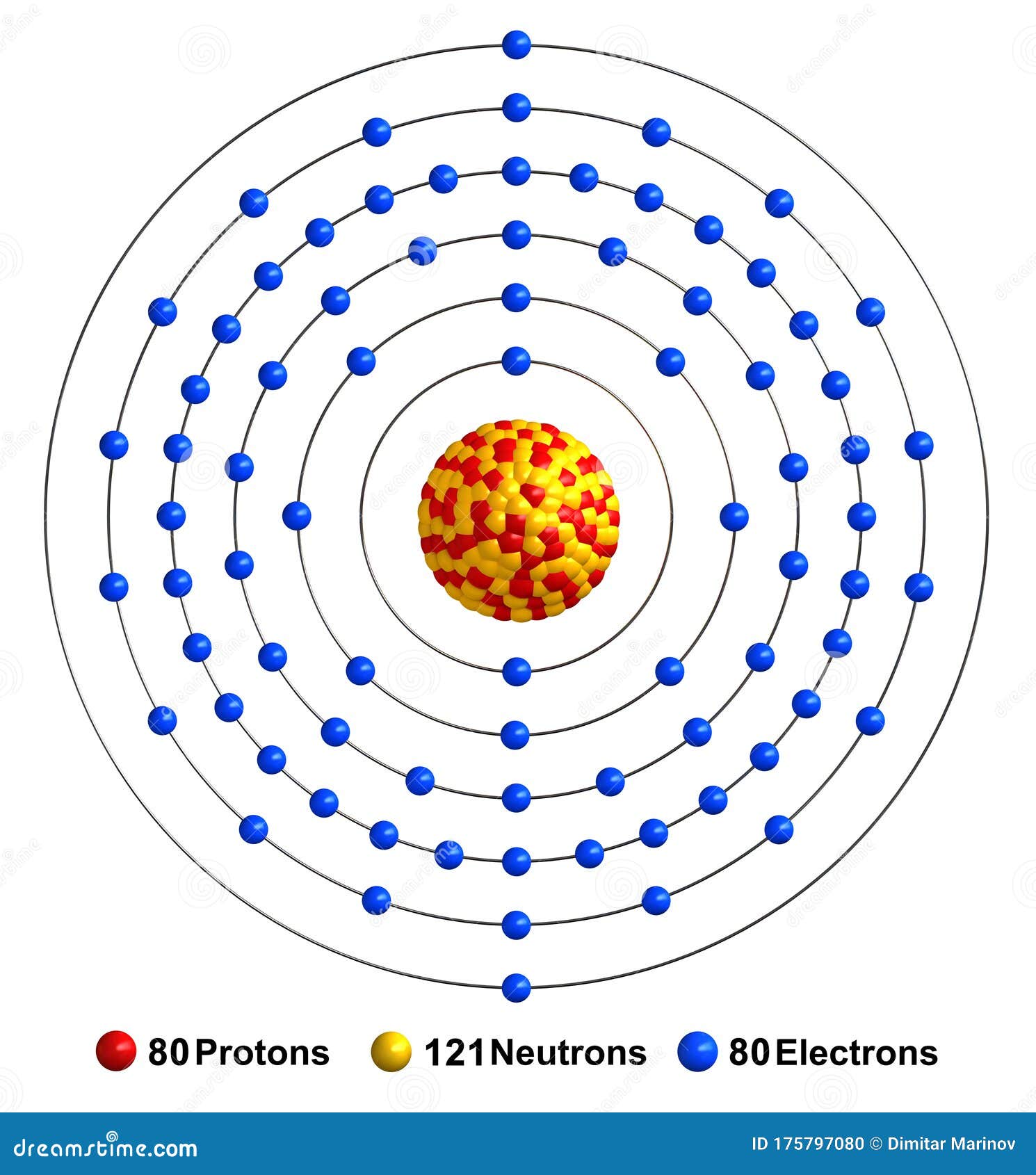
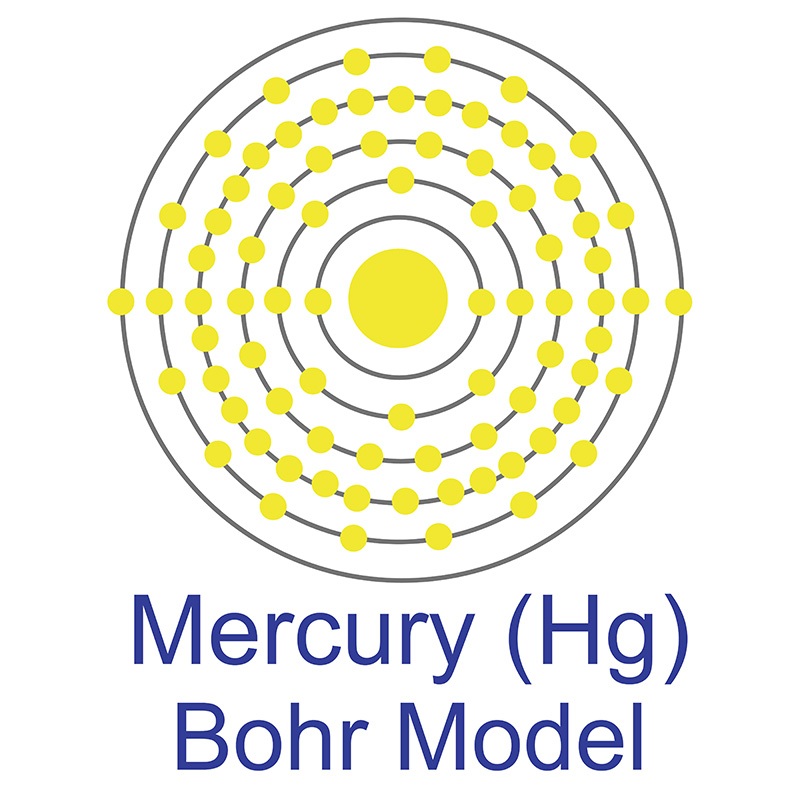
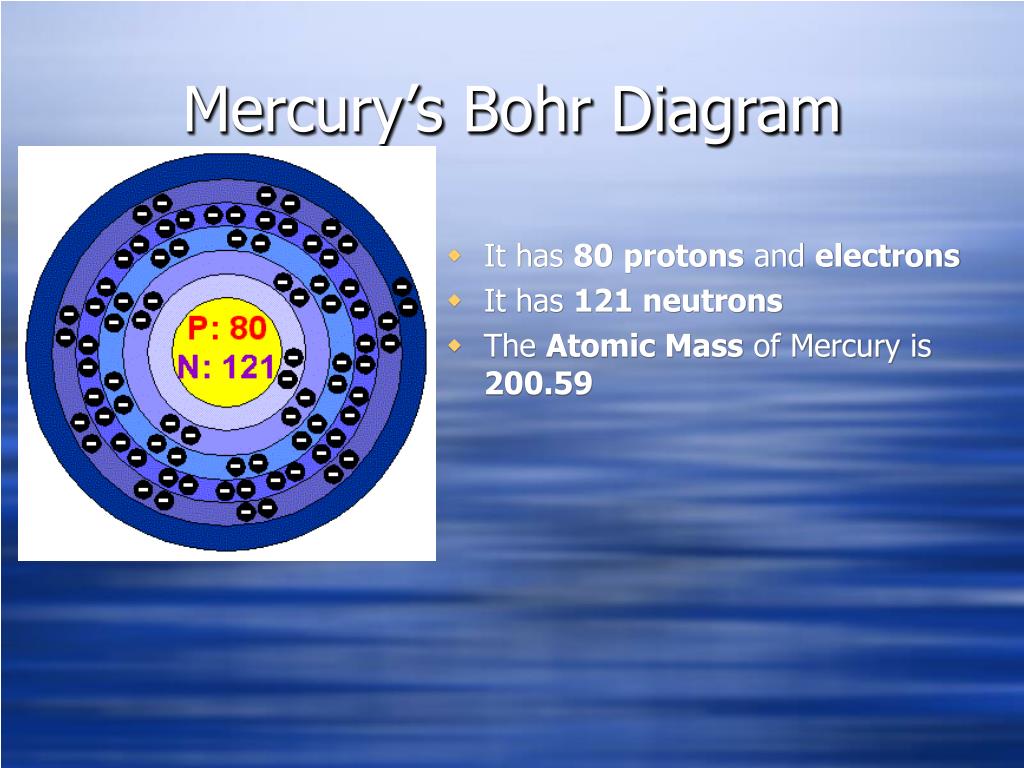
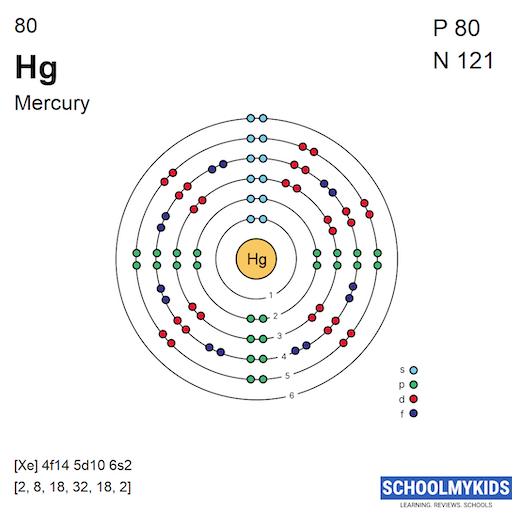
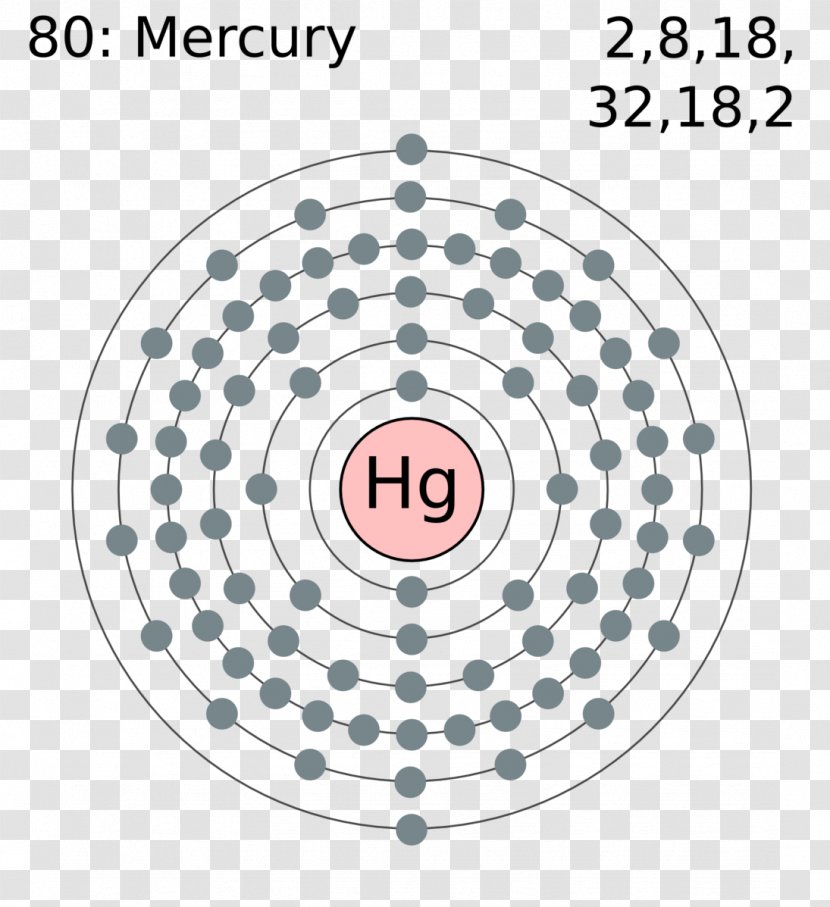
The Bohr Model of Mercury (Hg) has a nucleus that contains 121 neutrons and 80 protons. This nucleus is surrounded by six electron shells. The first shell of the Bohr diagram of Mercury has 2 electrons, the 2nd shell has 8, the 3rd shell has 18, the 4th has 32, the 5th has 18, and the 6th shell has 2 electrons. Also check -

Mercury, atomic structure Stock Image C013/1637 Science Photo Library
From the Bohr model, it can be found that the number of orbits or shells in mercury is 6. Hence, as mercury has 6 orbits, it lies in period 6 of the Periodic table. Why is Mercury in d-block? Before knowing this reason, first of all I want to ask you a simple question. How can you determine the blocks-wise position of elements?
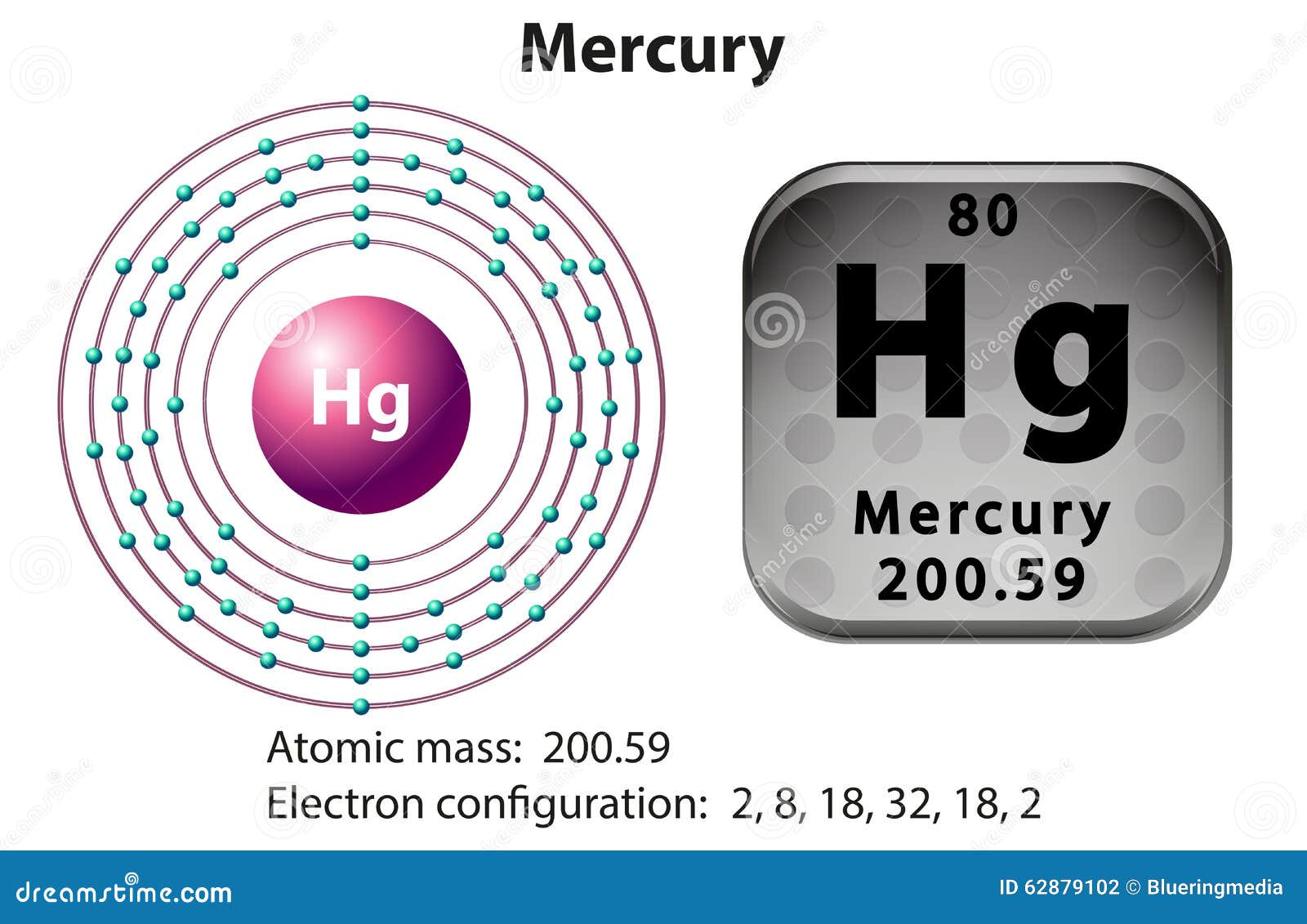
Symbol And Electron Diagram For Mercury Stock Vector Image 62879102
Mercury is a chemical element of the periodic table with chemical symbol Hg and atomic number 80 with an atomic weight of 200.592 u and is classed as a transition metal.. Bohr model: Electron shell for Mercury, created by Injosoft AB Hg. Figure: Shell diagram of Mercury (Hg) atom. Orbital Diagram. 1s: 2s: 2p: 3s: 3p: 3d: 4s: 4p: 4d: 4f: 5s.

What is the electron configuration of mercury
which is identical to the Rydberg equation in which R ∞ = k h c. R ∞ = k h c. When Bohr calculated his theoretical value for the Rydberg constant, R ∞, R ∞, and compared it with the experimentally accepted value, he got excellent agreement. Since the Rydberg constant was one of the most precisely measured constants at that time, this level of agreement was astonishing and meant that.